- Description
- Application
- Usage results (gallery)
- Usage protocol
- Staining mechanism
- Staining process (video)
- Publications
- Extras
Description
BioREE-B set is one of the BioREE™ series products for lanthanoid staining of biological objects, that provides the supravital tissue staining and allows visualizing on the scanning electron microscope (SEM) the structure of their subsurface layer, and their inner structure without preliminary fixation / dehydration / coating.
BioREE-B set with 2 staining stages for biological objects SEM study allows not only to obtain the high contrast image with the enhanced cell structure data, but also to evaluate its metabolic activity and comparative functional status.
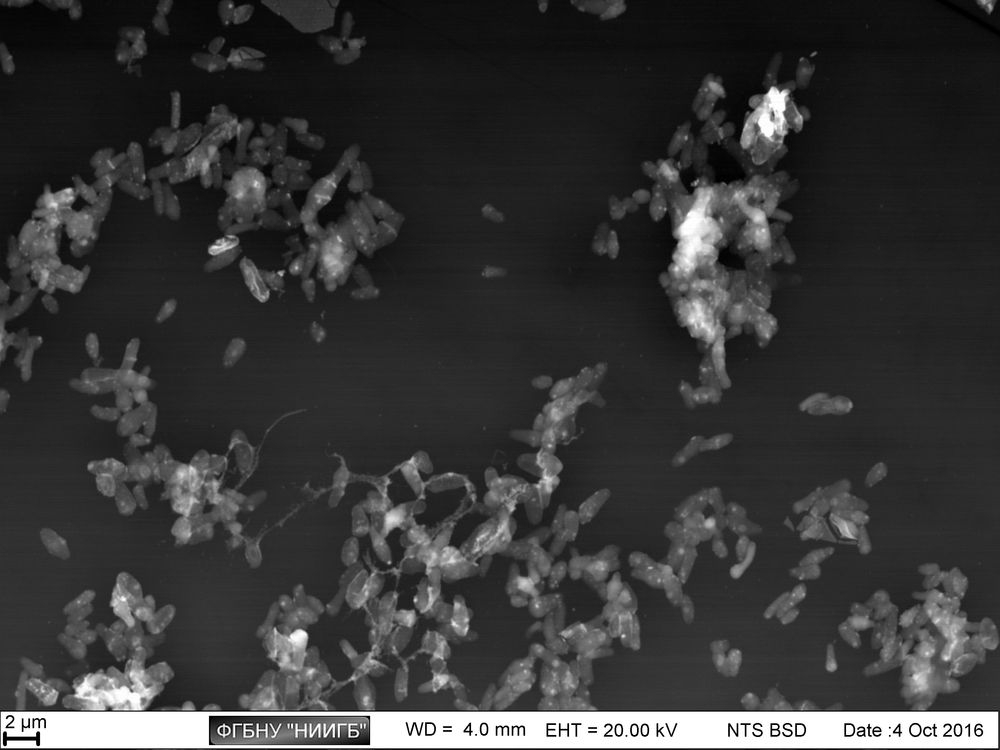
Due to adding a postfixation stage to the protocol, BioREE-B set is also applicable for studying the pathogenic microorganisms.
Application
BioREE-B reagent set is designed for biological sample preparation for scanning electron microscope (SEM) study. It is a lanthanoid contrast with an extra contrasting stage by lead salts, that provides the target substance saturation (supravital treatment) of the tissue, extracted from the organism and placed in the conditions that allow for the continuation of the basic metabolism; it also allows for SEM visualization of the surface structure, subsurface layer, and the inner structure of the biological sample as well as evaluating the comparative functional status of the sample cells. The concomitant result of contrasting is stabilizing natural cell dehydration, which prevents its deformation and helps preserve its native state during the study.
The set is applicable for the following kinds of biological objects:
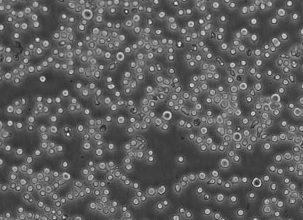
The contrasted samples obtained with the set are to be examined by the scanning electron microscope (SEM) in the low vacuum mode (55-90 Pa maintenance) and backscattered electrons (BSE) detector.
Limitations of use. Lanthanoid staining cannot be applied to fixed tissue blocks. Also, because of aggressive replacement of calcium by lanthanoids in the phosphates, it is not advisable to use the set for objects on phosphate scaffolds, and for teeth and bone tissue examination. An extra requirement for the sample carrier is its resistance to the mild acids, since the set includes a pH5 reagent.
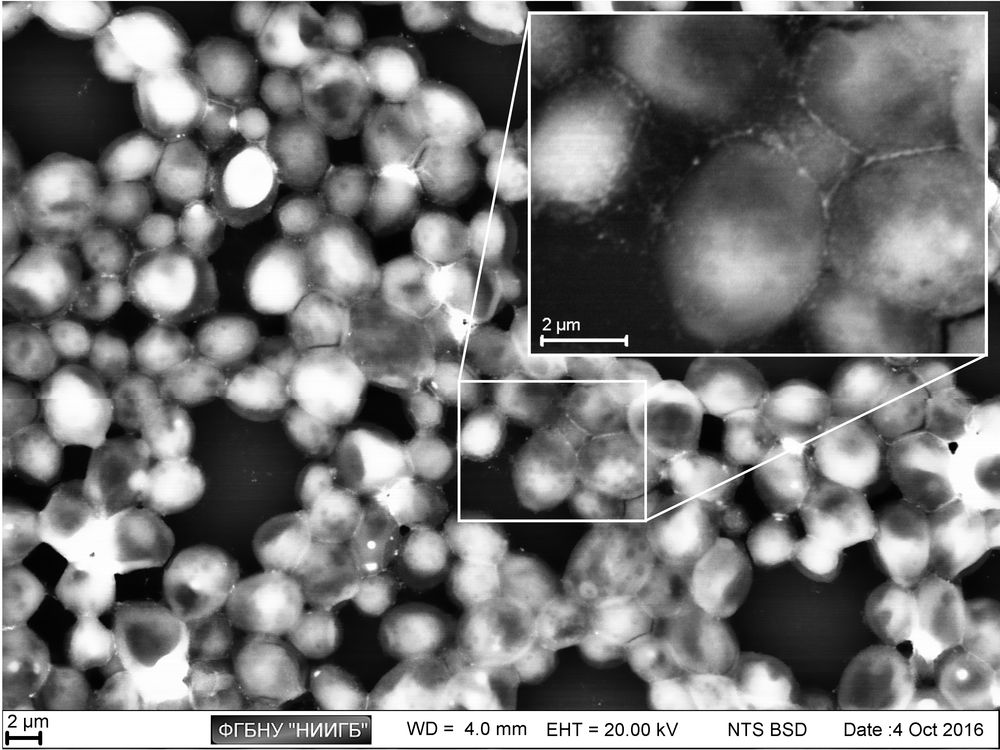
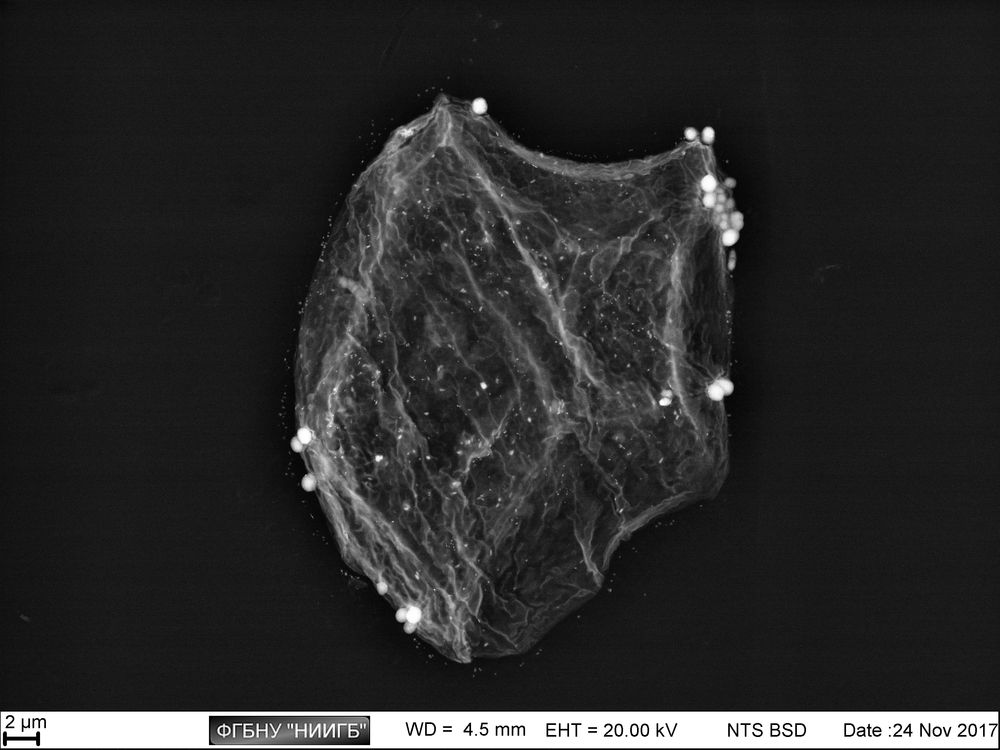
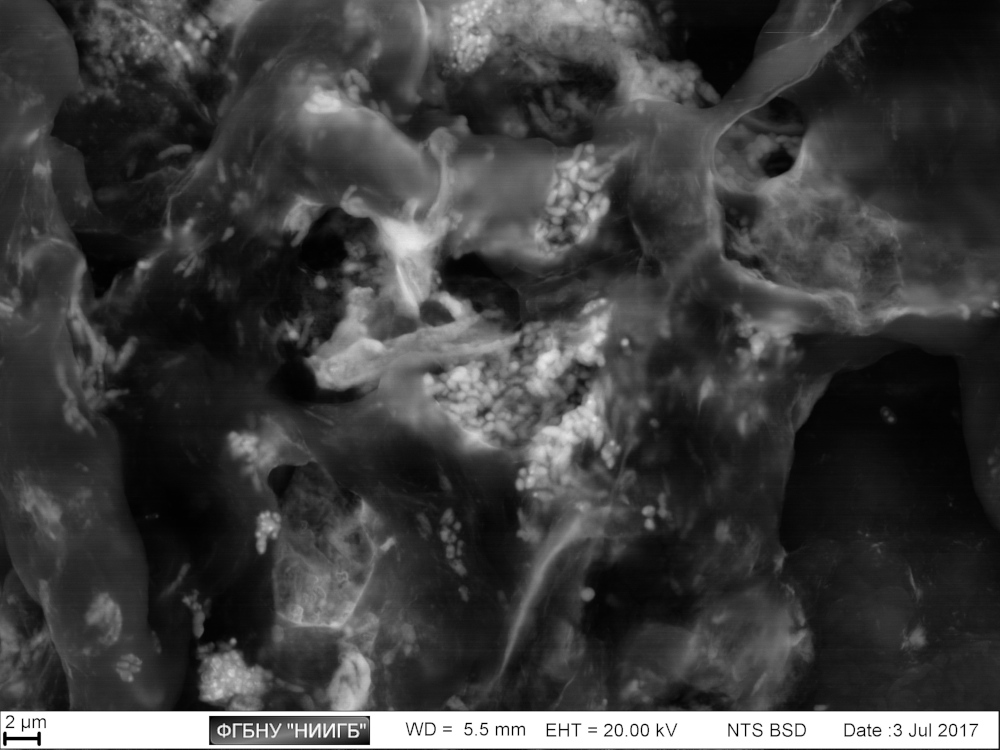
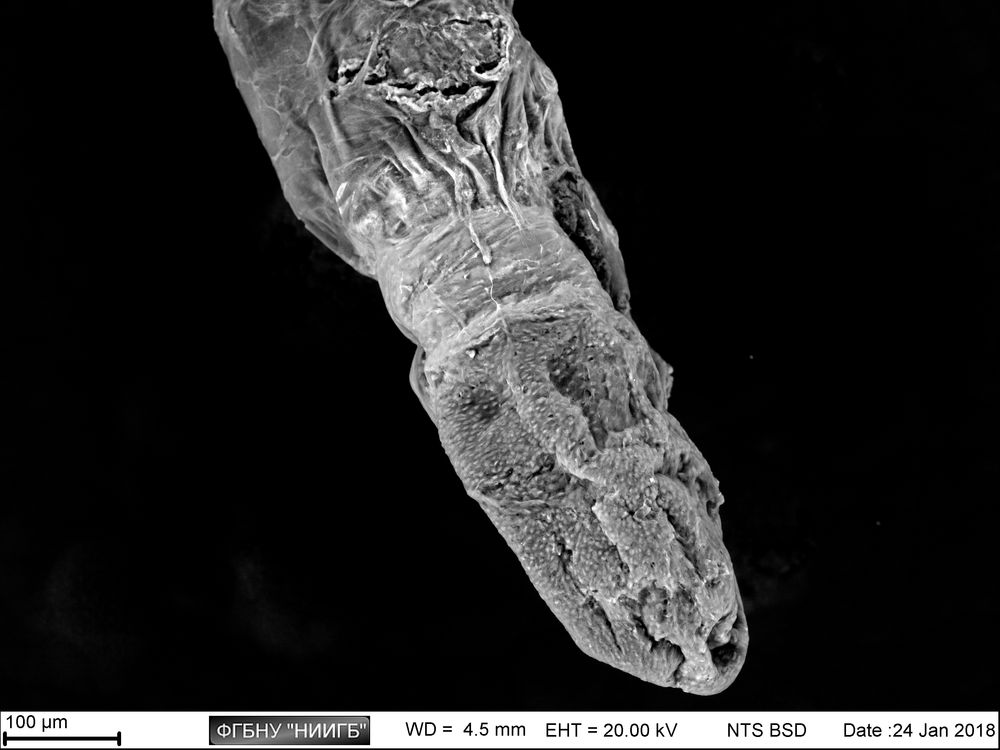
Usage results (gallery)
The study examples provided were obtained with the Zeiss EVO LS10 electron microscope in the Fundamental Research Laboratory of the Scientific Research Institute of Eye Diseases.
Usage protocol
BioREE-B set staining process requires less than an hour. If a prolonged sample storage is needed and/or if the research concerns pathogenic microorganisms, the set contains the extra stage of sample postfixation. The high informativity of contrasting is provided only with alive (non-fixed) cells.
Biological samples preparation
It is allowed to allocate the studied objects (thin tissue samples up to 1 mm, cultivated or primarily obtained cells, etc) on a plastic of any kind, silicate glass, including the coated one, and on the carbon and polymer scaffolds of non-phosphate basis. The sample carrier also has to be resistant to a mildly acid environment.
Analysis sequence:
- Primary sample washing with NaCl isotonic solution (required for removal of sorbed growth medium and fluids of main tissue substance with phosphate ions, and preventing their undesirable binding to staining lanthanoid and lead ions, which are tropic to phosphates).
- Sample placing into the isotonic water solution of neodymium chloride (or another Ln3+), with a 30 mins exposure.
Note: to obtain the best result, it is advised to perform staining in the most physiological conditions for the certain cell type. - First intermediate sample washing in the distilled water
(to remove the excess of the 1st staining substance). - Sample placing into the water isotonic solution of lead acetate, with a 15 mins exposure.
Note: is recommended to dip the samples which are attached to scaffolds into the solution upside down in order not to touch the vessel bottom with the sample surface. - Second intermediate sample washing in the distilled water
(to remove the excess of the 2nd staining substance). - Sample placing into the fixing solution
(This stage is required if a prolonged storage is needed and/or the pathogenic microorganisms are present in the sample). - Final sample washing in the distilled water (to remove the remains of the fixing reagent) for 1–10 sec.
When staining is done, excessive water is removed from the sample surface, and the sample is placed on a microscope stage. The scanning electron microscopy is then performed in low vacuum mode with 15–30 kV accelerating voltage, with backscattered electrons (BSE) detector.
Further information on the sample preparation, analysis and SEM visualization can be found in the following resources:
- BioREE-B user manual (eng / rus)
- Staining protocol (rus)
- SEM visualization protocol for the stained samples (rus)
Staining mechanism
Lanthanoid staining of living (non-fixed) cells is performed with low concentrations of its metal soluble salts (lanthanum chloride (LaCl3), neodymium (NdCl3), etc.), which instead of causing the immediate death of studied cells, slowly “shuts down” the energy demanding processes by the following reactions.
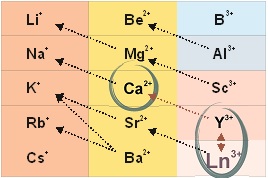
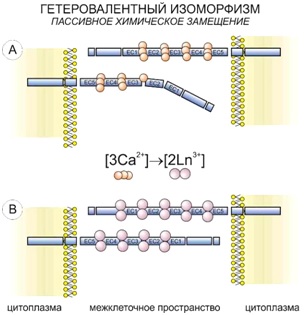
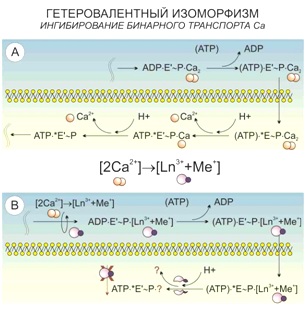
According to the principle of universal chemical isomorphism, as applied to the living systems, a heterovalent replacement of calcium ions happens in its structure when being treated with lanthanoid ions:
3Ca2+ ↔ 2La3+,
2Ca2+ ↔ La3+ + Me+.
In this case, an improvement of contrast is based not only on a higher atomic number of lanthanoid, compared to calcium, but also a higher efficacy of its binding both inorganic and organometallic substances.
One of the targets in the cells which undergo the heterovalent replacement is the calcium binding between five-member areas of cadherin (part of adhesive contacts).
The similar reactions also take place in the parts of cell membrane, where the calcium ATPases are located and the simultaneous transport of two calcium ions happens. The system captures lanthanoid paired with alkali metal, and in the bifurcation point with independent Ca2+ transport the lanthanoid is no longer able to leave it as it remains bound to ATP.
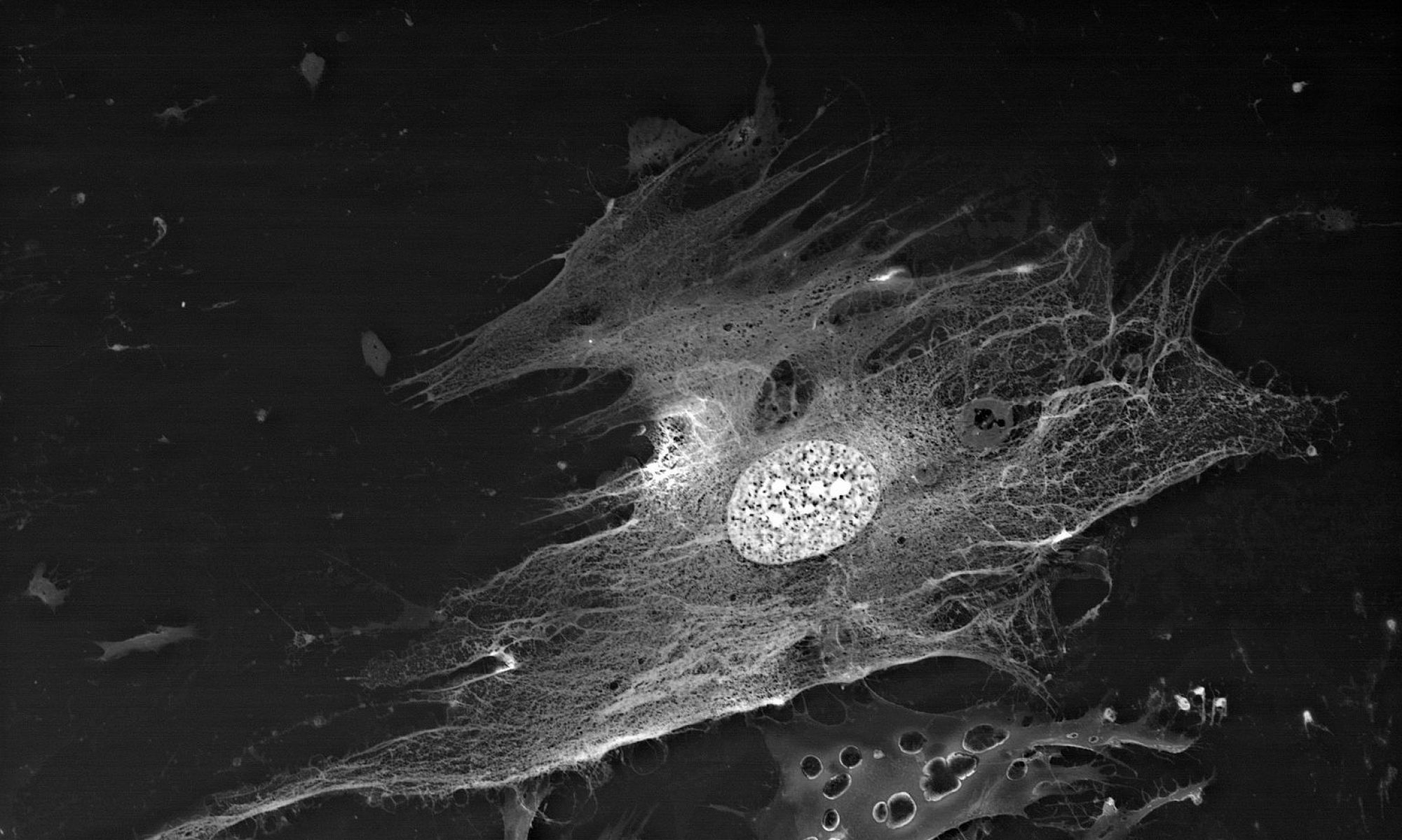
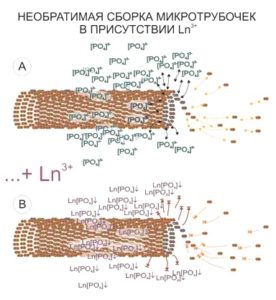
The areas of cytoskeleton locations present another target of lanthanoid accumulation in the cell. It has to be noted that the size of visualized structures will exceed the actual microtube size. This is supposed to be a “halo” of a bound phosphate anions around the cytoskeleton elements, which have been released during the microtubes assembly and mark brightly the microtubes location and/or statistical directions of their assembly by producing the insoluble connections with lanthanoid ions.Thus just the first stage of BioREE-B staining enhances the contrast of the SEM image more than twofold.
Application of the lead acetate water solution as the 2nd stage of BioREE-B staining enhances the contrast of the images obtained even further due to the following reactions:
А) Reaction of lanthanoid displacement by lead in the simple phosphates by the lowest solubility principle:
3Pb2+ + 2Ln[PO4]↓ → 2Ln3+ + Pb3[PO4]2↓
при ПР(Ln[PO4]) = 3.7·10 – 23;
ПР(Pb3[PO4]2) = 7.9·10 – 43.
It has to be noted that instead of two lanthanoid cations there would be three lead cations per two phosphate anions. This reaction mostly increases the brightness of the backscattered electrons of cytoskeleton parts and mitochondriae since they are spatially bound with more free phosphate anions. Thus, when enhancing the staining with lead acetate, the phosphate binding sites appear to be significantly more contrast.
B) Reaction of glucosides interaction with lead acetate, analogous to the Barfed reaction with the copper cations:
CH2OH(CHOH)4COH + Pb(CH3COO)2 + 2H2O ⇒ CH2OH(CHOH)4COOH + PbO↓ + 4CH3COOH .
These reactions in the cell and extracellular matrix target glucose, phospholipids and biochemical elements which participate in the cell energy supply, located mostly in cytoplasm and endoplasmatic reticulum. The contrast of these areas is enhanced by lead oxide production.
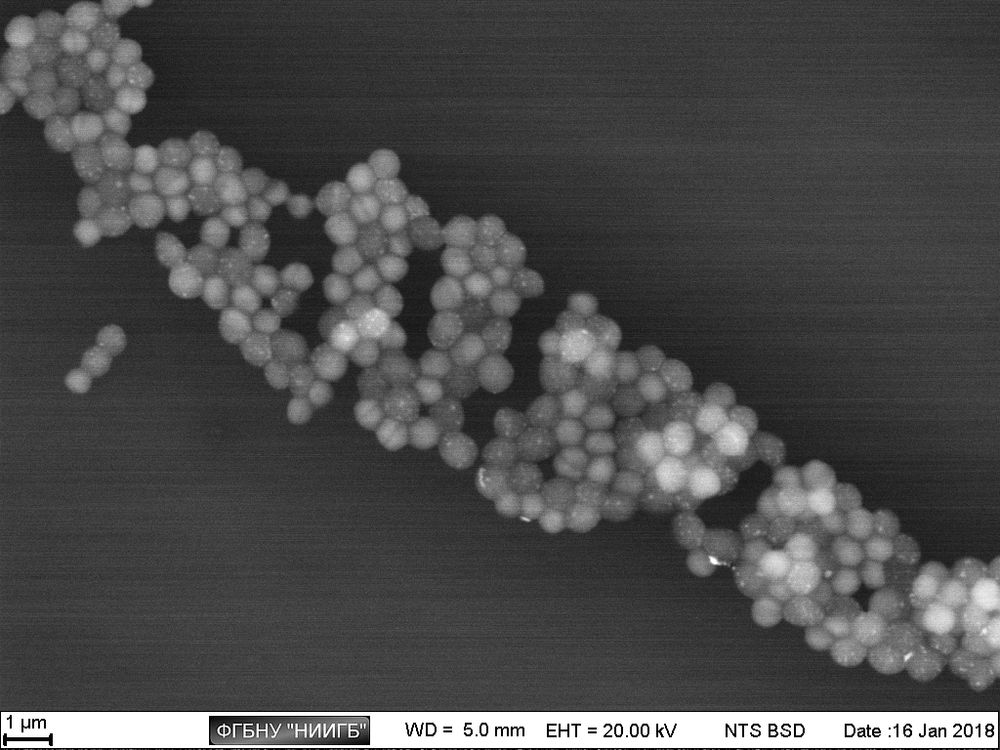
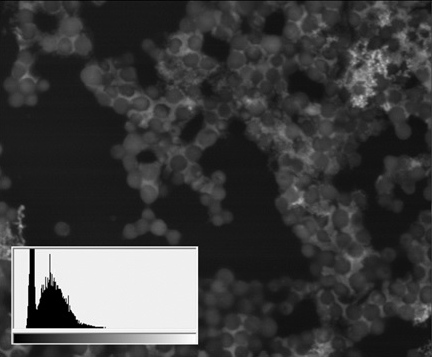
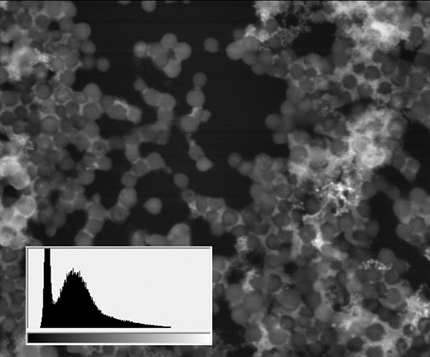
Staphylococcus aureus culture АТСС 29213, SEM image, BSE mode
(18 μm view field; the sidebars: histograms of brightness distribution frequencies, created for a corresponding image; accelerating voltage 20 kV, low vacuum (70 Pa), 120 pA current)
Thus, apart from the main structures, visible because of specific lanthanoid accumulation in the cell (nucleoli, intercellular contact areas, location of calcium pumps and remnants of cytoskeleton assembly in the cell membrane), the final staining stage with lead gives us an access to a whole group of visible structures, responsible for the energetic condition of a cell. It also allows for performing a comparative analysis of metabolic activity in different kinds of cells within one sample.
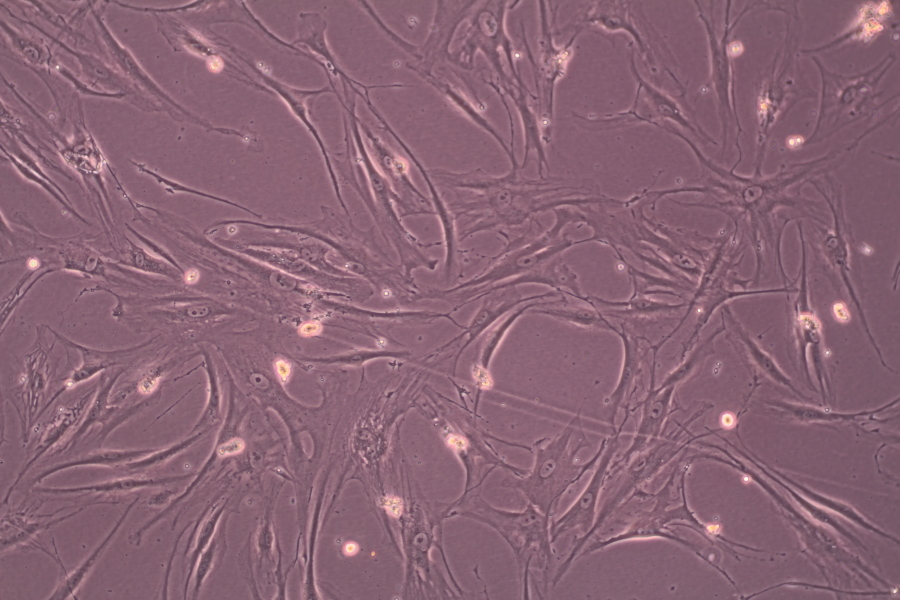
A mixed culture of human corneal stroma cells and A549 line cells (human lung carcinoma):
cancer cells on the image are stained more intensively due to their bigger metabolic activity
Staining process
RU 2672356 C1 Patent
http://www1.fips.ru/wps/PA_FipsPub/res/Doc/IZPM/RUNWC1/000/000/002/672/356/%D0%98%D0%97-02672356-00001/document.pdf
International patent application № PCT/RU2019/000119 (от 22/02/2019)
https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2019177492&_cid=P20-K1G5WZ-12570-1
Publications
- Consequent marking of biological object ultrastructure with neodymium and lead in a two-stage preparation method for scanning electron microscope / I.A. Novikov, A.M. Subbot, I.V. Chebotar, O.A. Pak // Analitika 2018, Vol. 8, N 4(41), pp. 358–363.
DOI: 10.22184/2227-572X.2018.41.4.358.363
http://www.j-analytics.ru/journal/article/6981 - Patent 2018108571, 12.03.2018. Method of biological sample preparation for the scanning electron microscopy study / I.A. Novikov, A.M. Subbot, I.V. Chebotar, O.A. Pak, A.I. Bursov, A.V. Sitnikov // Russian Patent N 2672356, 2018. Bulletin #32.
http://www1.fips.ru/wps/PA_FipsPub/res/Doc/IZPM/RUNWC1/000/000/002/672/356/%D0%98%D0%97-02672356-00001/document.pdf - Fast and easy visualization method of impression cytology probe with microbiota detection on the ocular surface / Yugay N.M., Novikov I.A., Subbot A.M., Khalatyan K.S. // 26th International Student Congress Of (bio)Medical Sciences, Groningen (Netherlands), 2019, p. 412.
https://www.researchgate.net/publication/333320273_Fast_and_easy_visualization_method_of_impression_cytology_probe_with_microbiota_detection_on_the_ocular_surface - A rapid method of whole cell sample preparation for scanning electron microscopy using neodymium chloride / Chebotar I.V., Novikov I.A., Subbot A.M., Mayansky N.A. // Micron 2019, 124. DOI: 10.1016/j.micron.2019.102687
https://www.sciencedirect.com/science/article/pii/S0968432818303111