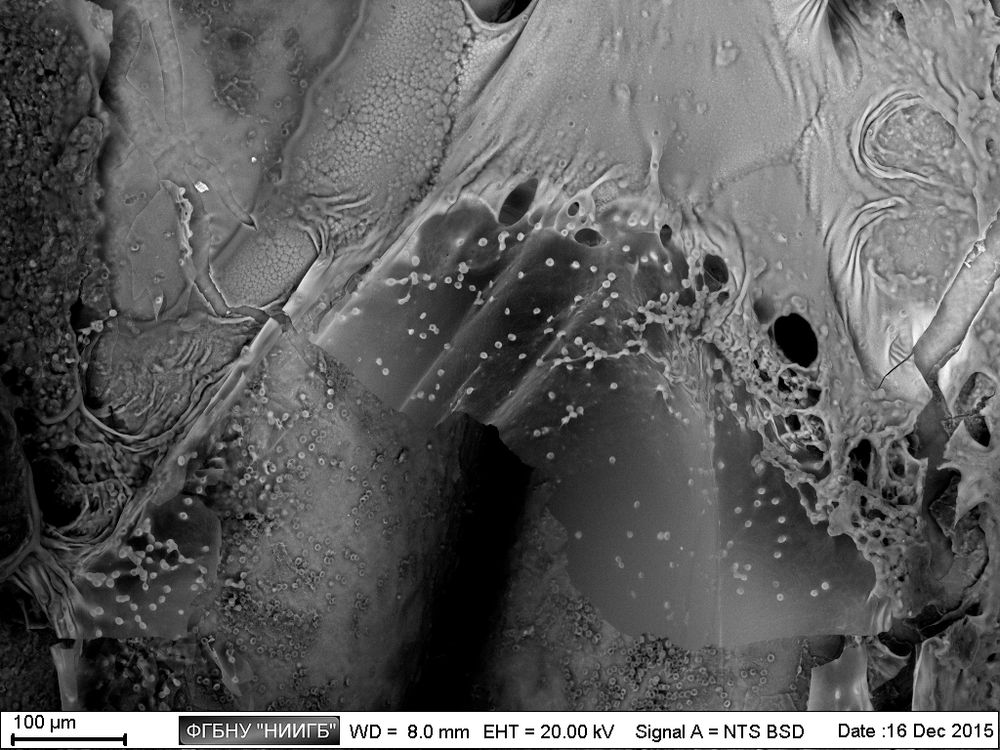
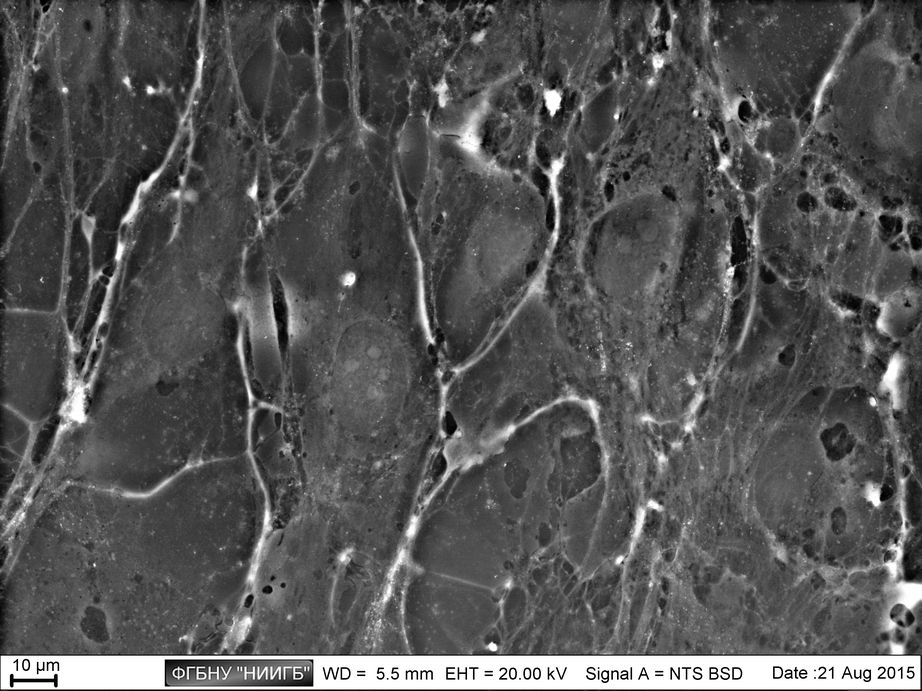
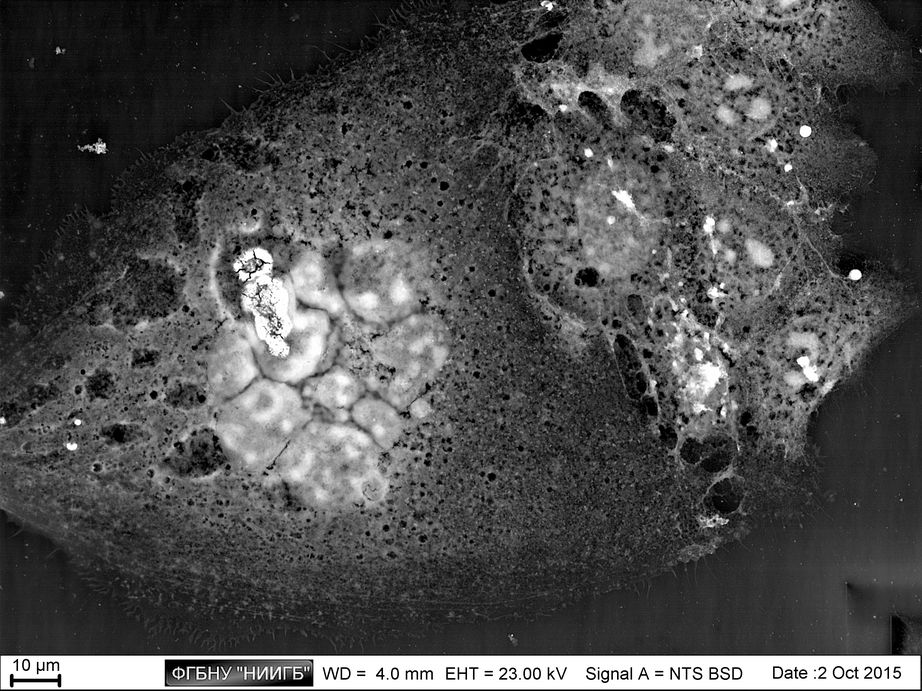
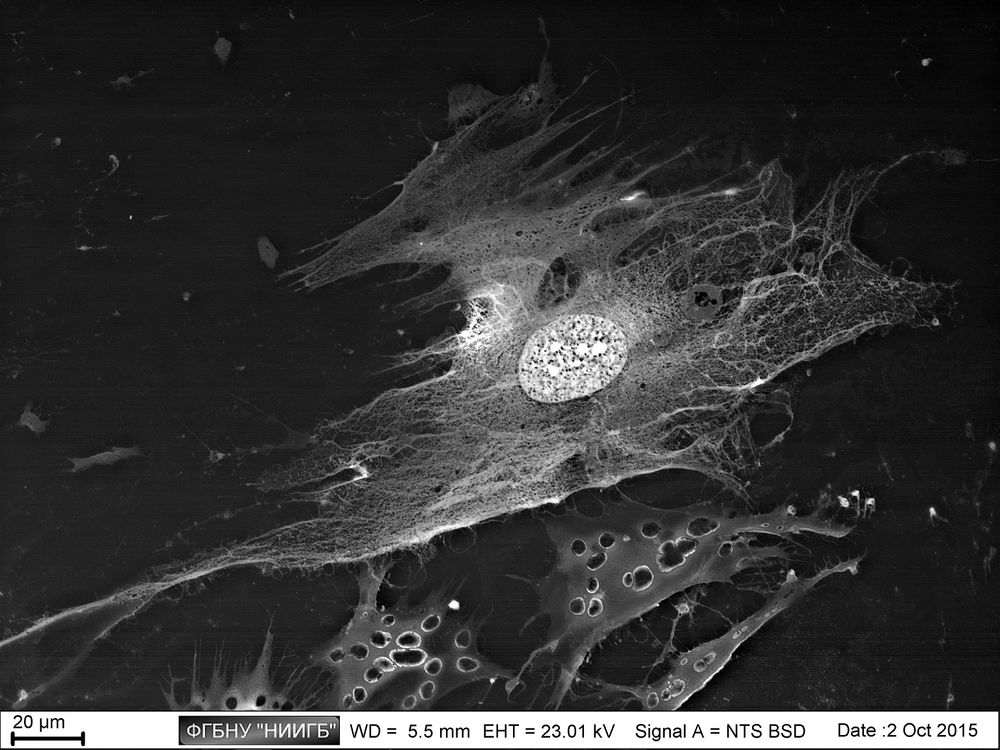
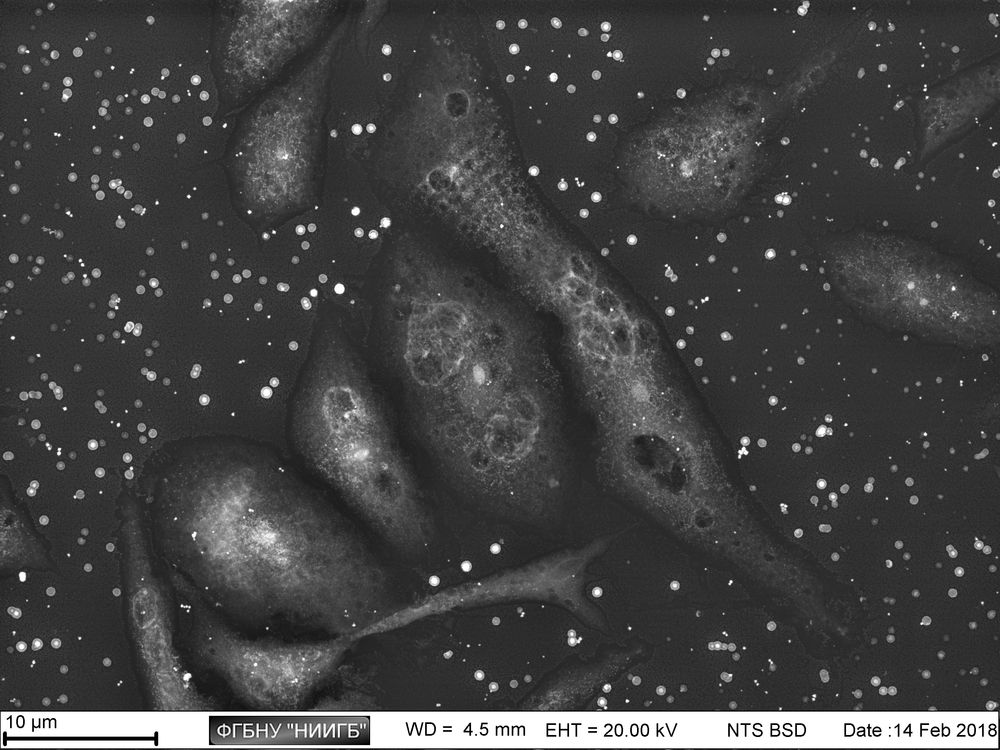
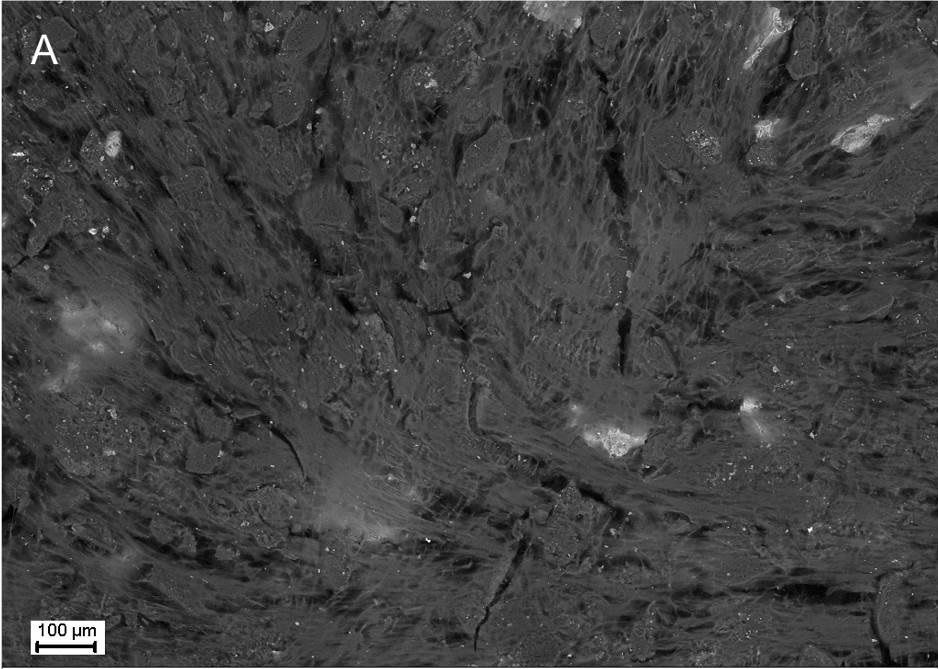
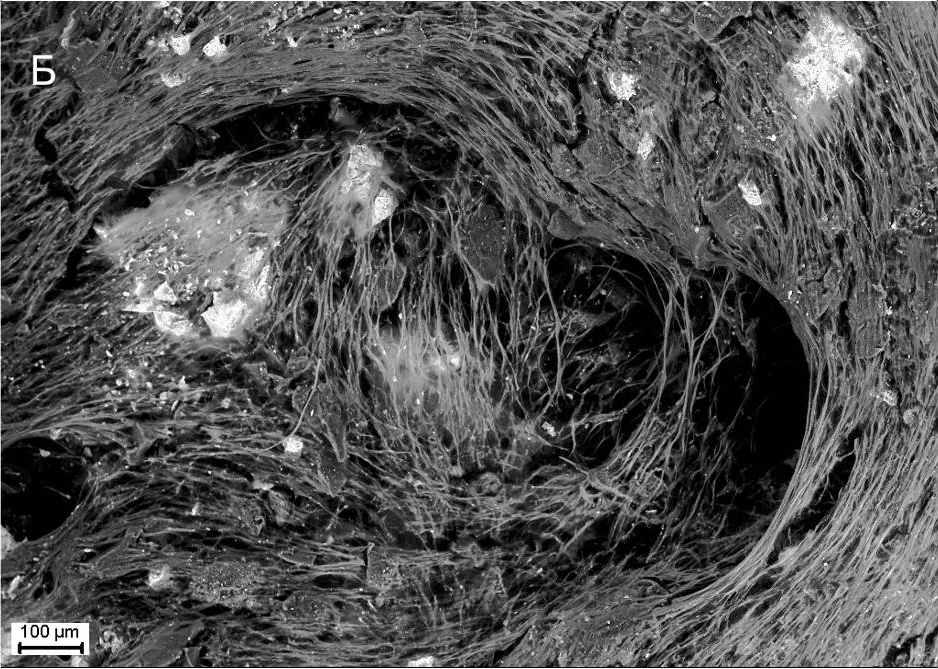
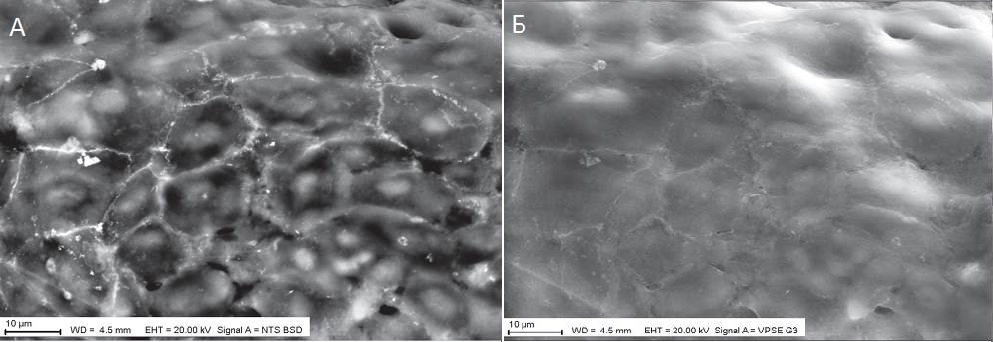
Description
BioREE-A set is a product of BioREE™ series for lanthanoid staining of biological objects, providing supravital treatment of cells and tissues and allowing visualizing via scanning electron microscope (SEM) the subsurface layer structure of biological samples, as well as their inner structure without preliminary fixation, dehydration and coating.
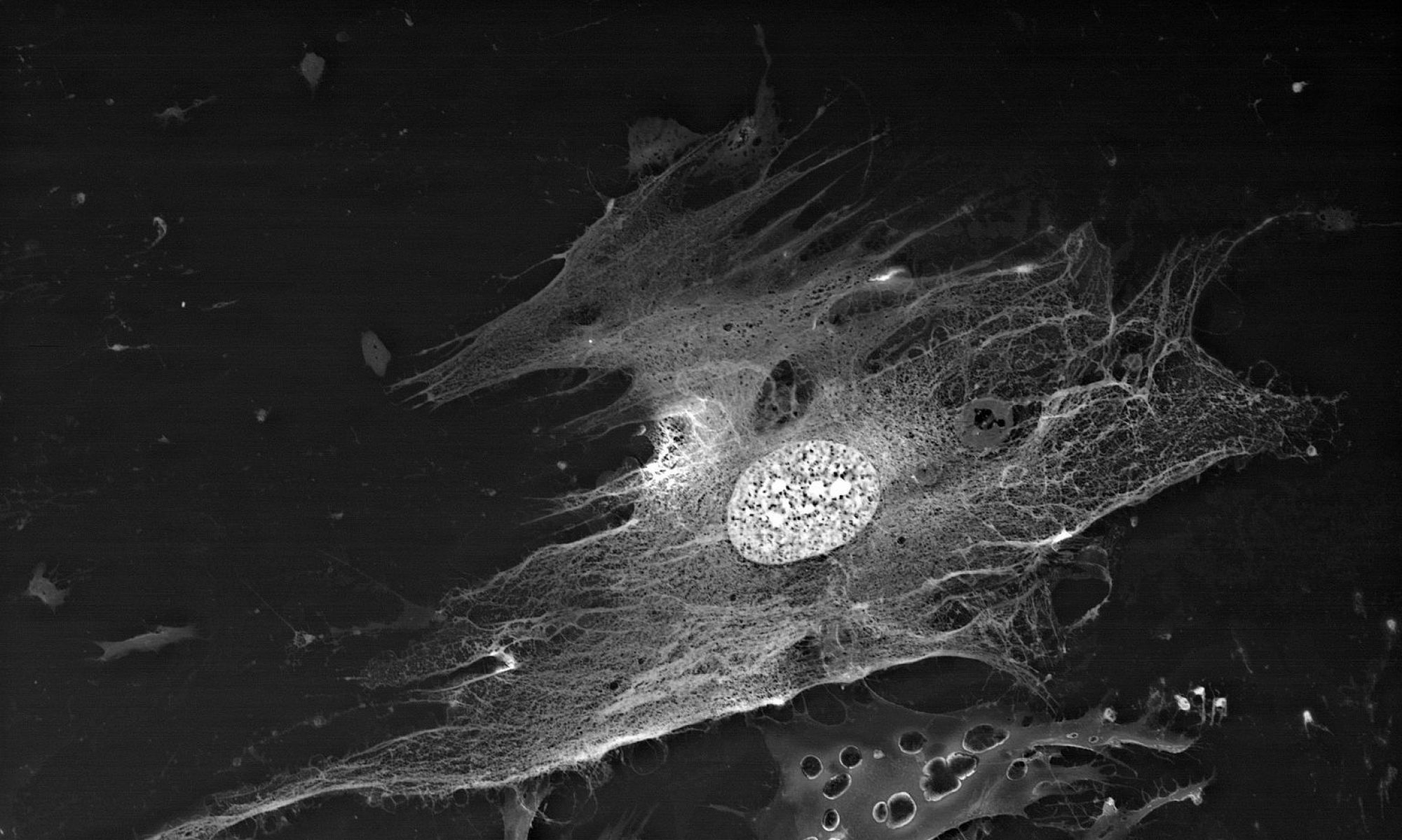
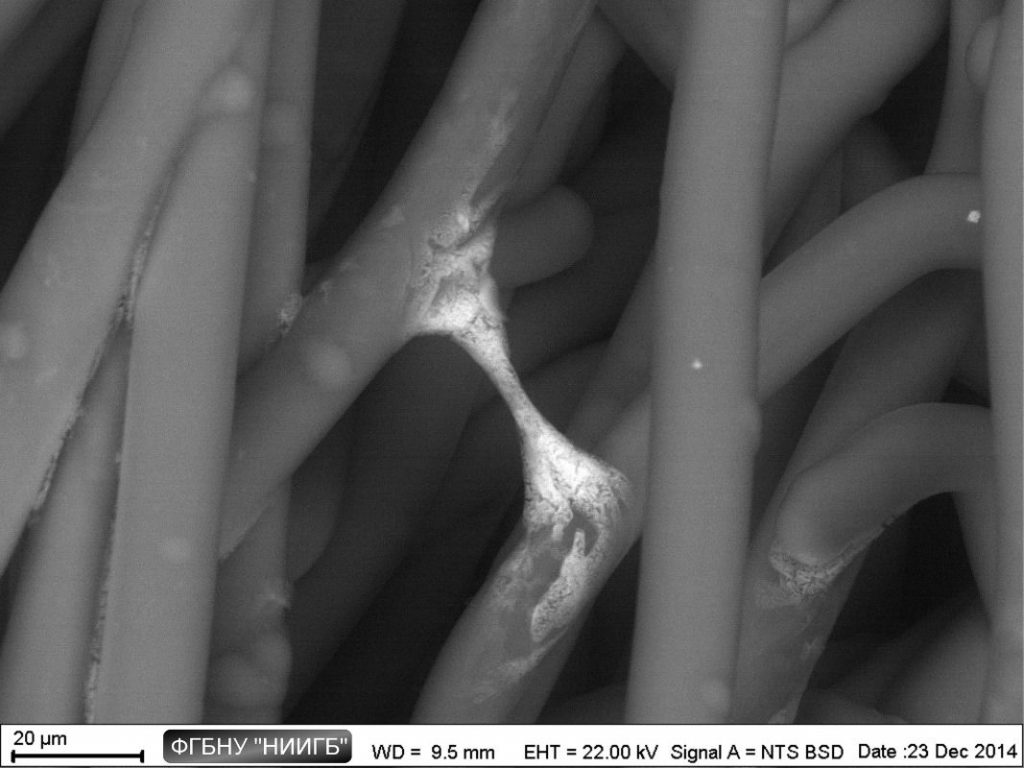
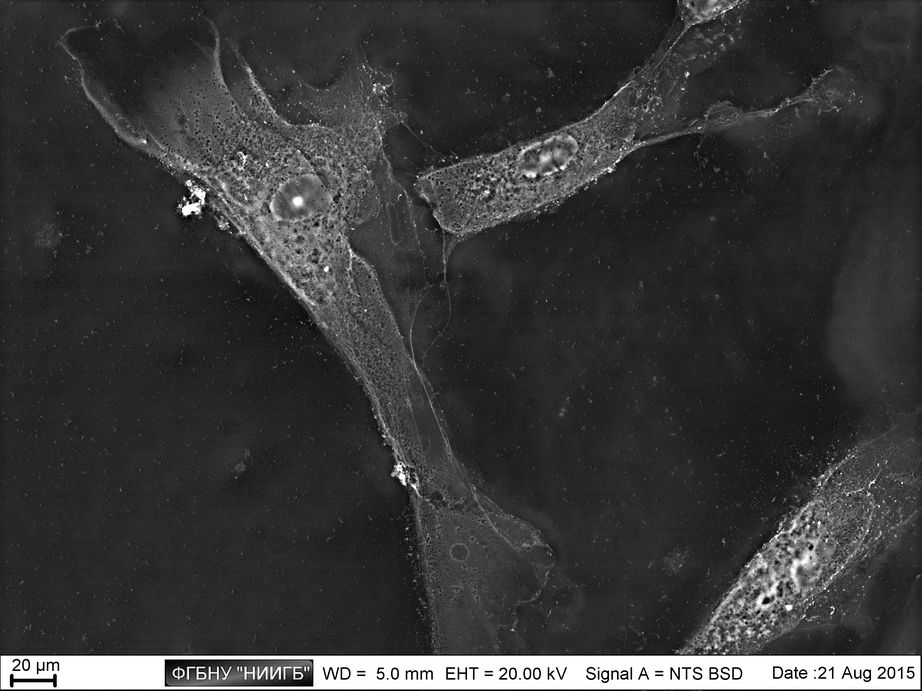
Biological objects SEM study with BioREE-A set allows saving the native object condition as much as possible when obtaining the high-contrast image with extended data about cell structure.
UpApplication
BioREE-A reagent set is designed for biological sample preparation for scanning electron microscope (SEM) study. It is a lanthanoid contrast that provides the target substance saturation (supravital treatment) of the tissue, extracted from the organism and placed in the conditions that allow the continuation of the basic metabolism; it also allows for SEM visualization of the surface structure, subsurface layer, and the inner structure of the biological sample. The concomitant result of contrasting is stabilizing natural cell dehydration, which prevents its deformation and helps preserve its native state during the study.
The set is applicable for the following kinds of biological objects:
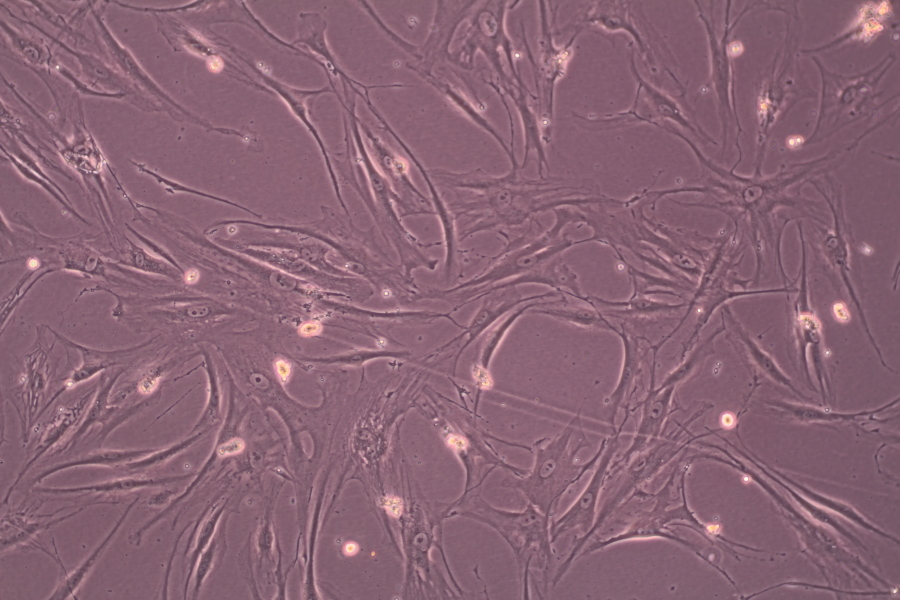
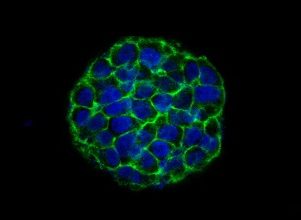
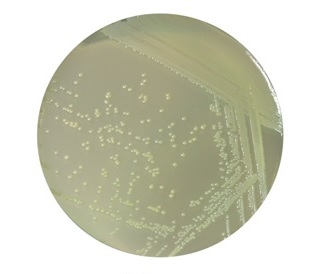
The contrasted samples obtained with the set are to be examined on the scanning electron microscope (SEM) in low vacuum mode (55-90 Pa maintenance) and backscattered electrons (BSE) detector.
Limitations of use. Lanthanoid staining cannot be applied to fixed tissue blocks. Also, because of aggressive replacement of calcium by lanthanoids in the phosphates, it is not advisable to use the set for objects on phosphate scaffolds, and for teeth and bone tissue examination.
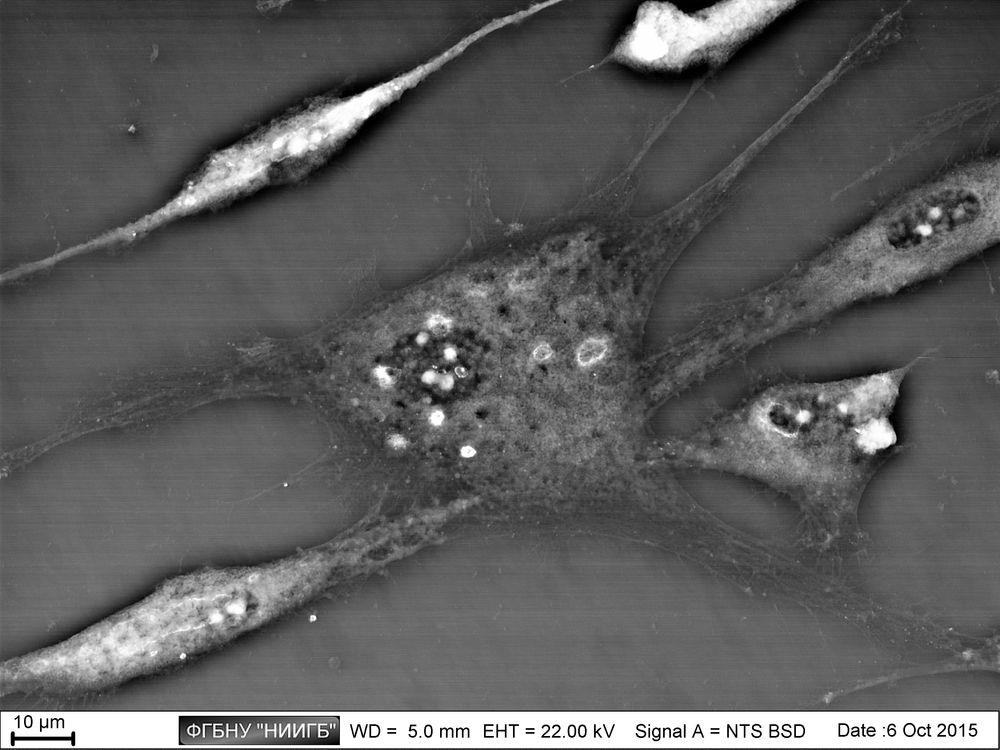
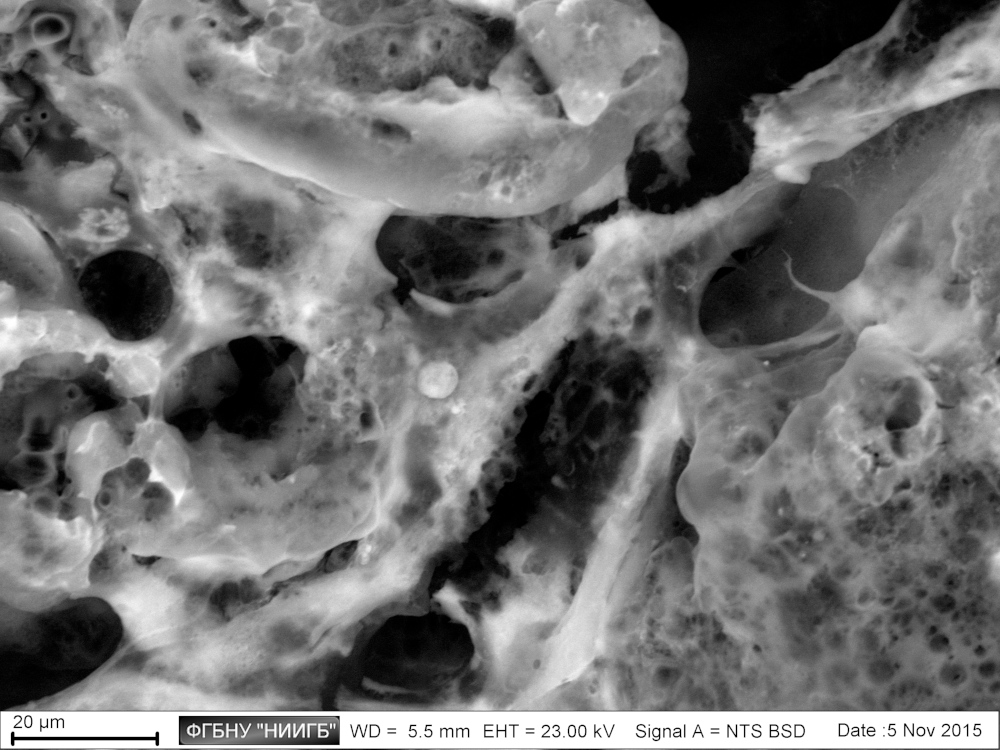
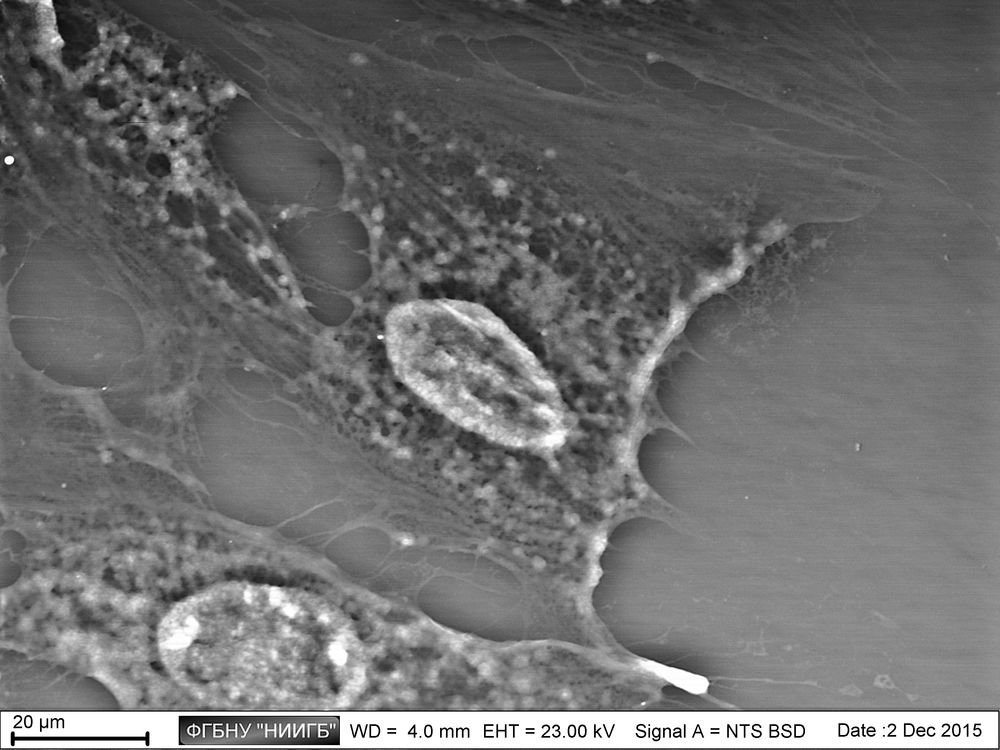
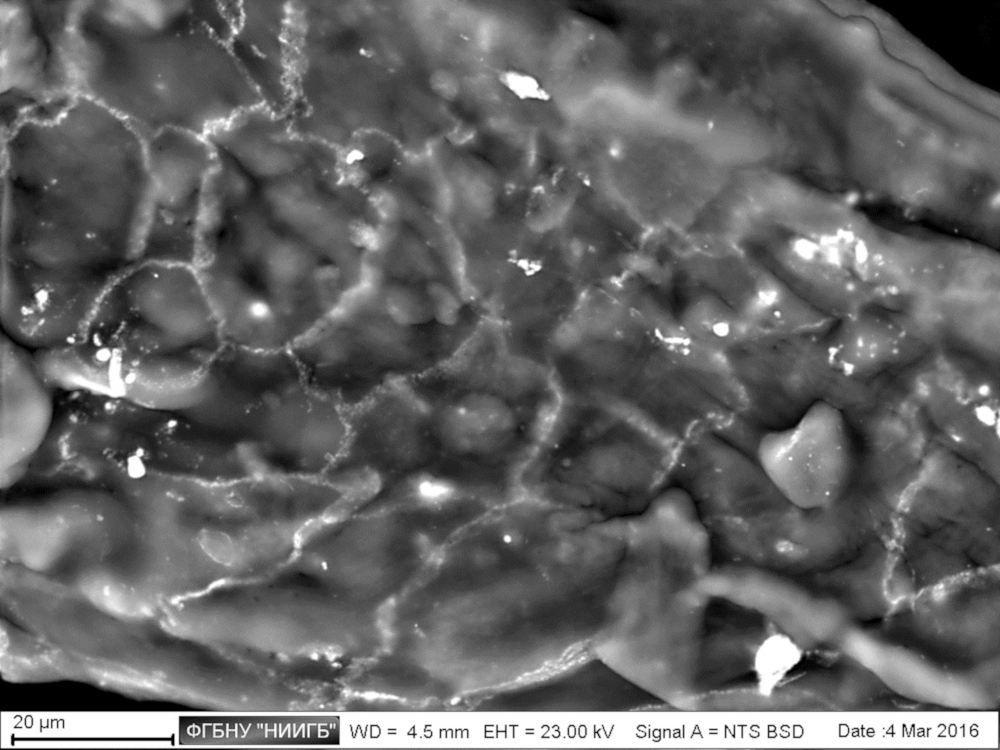
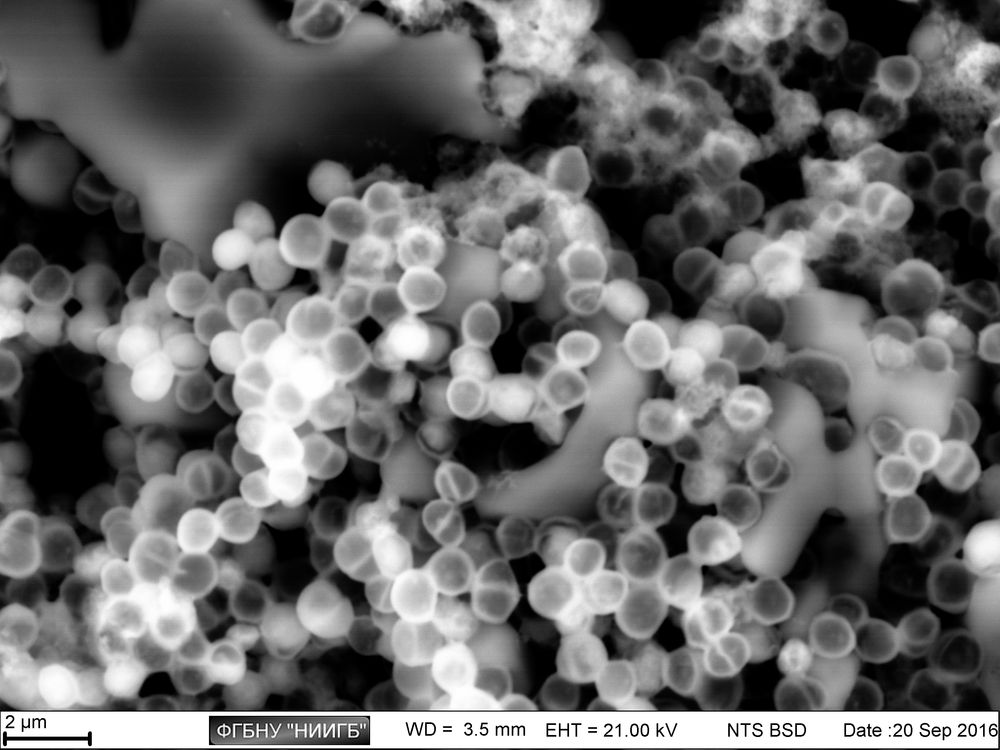
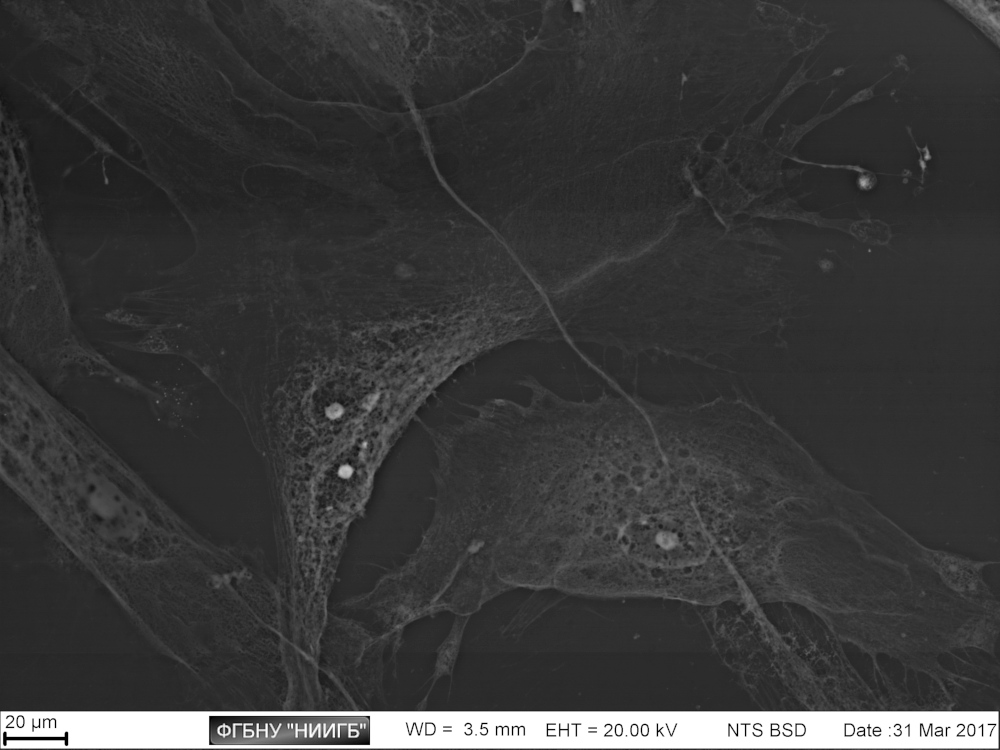
UpResults (gallery)
UpThe given study examples were obtained by means of the Zeiss EVO LS10 electron microscope in the Fundamental Research Laboratory of the Scientific Research Institute of Eye Diseases.
Usage protocol
Depending on the type of studied sample, BioREE-A staining process may require from 0.5 to 1.5 hours. High contrasting informativity is provided only when using alive (non-fixed) cells as a studied sample.
Biological samples preparation:
It is allowed to allocate the studied objects (thin tissue samples up to 1 mm, cultivated or primarily obtained cells, etc) on a plastic scaffold of any kind, silicate glass, including the coated one, and on carbon and polymer scaffolds of non-phosphate basis.
Analysis sequence:
- Primary sample washing with NaCl isotonic solution (required for the removal of sorbed growth medium and fluids of main tissue substance with phosphate ions, and preventing their undesirable binding to staining lanthanoid ions).
- Sample placing into the isotonic water solution of neodymium chloride (or another Ln3+), with a 15-45 mins exposure, depending on the sample type:
| Sample type | Exposure, mins |
| Cells on various scaffolds: | |
| Separate cells on plastic or adhesive glass | 15 |
| Cell monolayer on plastic or adhesive glass | 30 |
| Tissue engineering structure, 3D scaffold | 30 |
| Native tissue | 45 |
| Suspensive cells: | |
| Cell suspension | 15 |
| Cells precipitated on a membrane | 15 |
| Bacteria, protozoa, yeast etc | 15 |
Note: to obtain the best result, it is advised to perform staining in the most physiological conditions for the certain cell type.
3. Final washing of a sample with distilled water (for removing the staining reagent remnants) for 1–10 sec.
When staining is done, excessive water is removed from the sample surface, and the sample is placed on a microscope stage. The scanning electron microscopy is then performed in low vacuum mode with 15–30 kV accelerating voltage, with backscattered electrons (BSE) detector.
Work with cell suspensions: special features
Before staining, the cell suspension has to be centrifuged (200–1000g for the animal cells, 5000–15000g for bacteria) with subsequent supernatant removal. When the first washing solution is added, the sample is shaken with the vortex or stirred by pipetting, centrifuged again and left resuspended in 1ml of neonatant, removing the excess suspension voume.
After sample exposure to lanthanoid solution, the cells are precipitated on a syringe filter membrane. The membrane filter cassette is washed by distilled water and then the membrane with precipitated cells is quickly removed and placed onto the microscope stage.
Further information on the sample preparation, analysis and SEM visualization can be found in the following resources:
- BioREE-A user manual (rus / eng)
- Staining protocol (rus)
- SEM visualization protocol for the stained samples (rus)
Staining mechanism
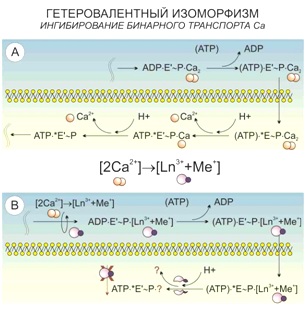
Lanthanoid staining of living (non-fixed) cells is performed with low concentrations of its metal salts (lanthanum chloride (LaCl3), neodymium (NdCl3), etc.), which instead of causing the immediate death of studied cells, slowly “shuts down” the energy demanding processes by the following reactions.
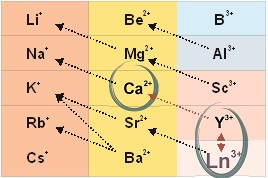
According to the principle of universal chemical isomorphism, as applied to the living systems, a heterovalent replacement of calcium ions happens in its structure when being treated with lanthanoid ions:
3Ca2+ ↔ 2La3+,
2Ca2+ ↔ La3+ + Me+.
This enhances the contrast of the SEM image obtained in the BSE mode more than twofold.
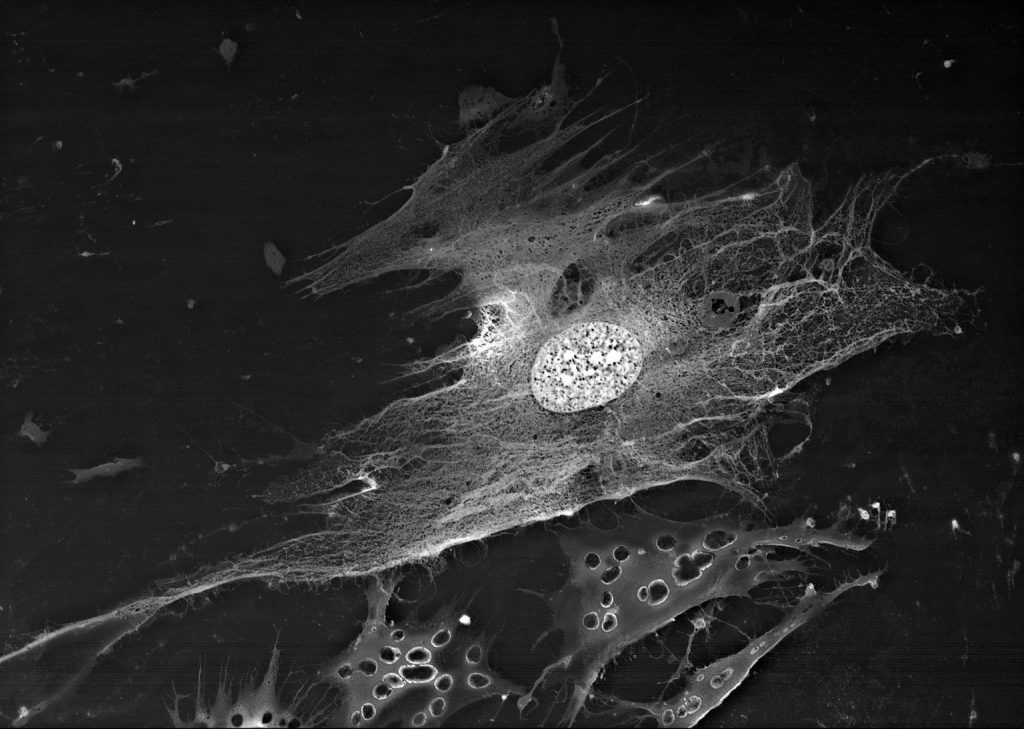
SEM image in BSE mode of a polylactoglycolide scaffold, colonized by MMSC, 2 days after colonization
Such an improvement of contrast is based not only on a higher atomic number of lanthanoid, compared to calcium, but also a higher efficacy of its binding both inorganic and organometallic substances.
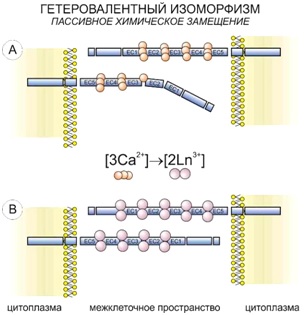
One of the cell targets undergoing the heterovalent replacement is the calcium binding between five-member areas of cadherin (part of adhesive contacts).
Similar reactions also take place in the parts of cell membrane, where calcium ATPases are located and the simultaneous transport of two calcium ions happens. The system captures lanthanoid paired with alkali metal, and in the bifurcation point with independent Ca2+ transport the lanthanoid is no longer able to leave it as it remains bound to ATP.
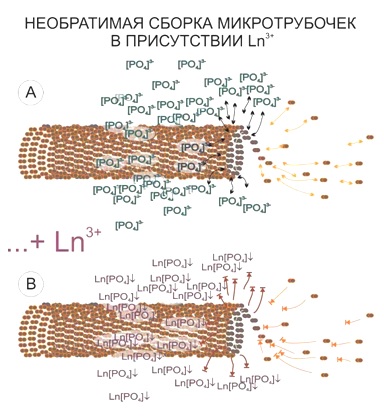
The areas of cytoskeleton locations present another target of lanthanoid accumulation in the cell. It has to be noted that the size of visualized structures will exceed the actual microtube size. This is supposed to be a “halo” of a bound phosphate anions around the cytoskeleton elements, which have been released during the microtubes assembly and mark brightly the microtubes location and/or statistical directions of their assembly by producing the insoluble connections with lanthanoid ions.
Particularly high-contrast SEM images of the samples made with BioREE-A can be obtained with a backscattered electrons (BSE) detector, apart from secondary electron (SE) detector, traditionally used in electron microscopy.
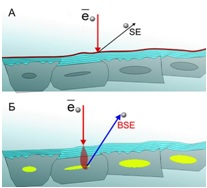
This way, a specific accumulation of lanthanoids in cell structures during BioREE-A staining with a following SEM study in BSE mode provides the visualization of the following cell parts:
- nucleoli (due to lanthanoid binding with RNA),
- intercellular contacts area (due to calcium replacement by lanthanoid in the cadherin-like proteins),
- calcium pumps location (trivalent lanthanoid ion coupled with an alkali metal replaces the paired transport of two calcium ions),
- remnants of the cytoskeleton assembly (phosphate remnants, produced during the energy consuming processes, like the microtube assembly, are sedimentated with the lanthanoids into the insoluble form).
Publications
- Supravital lanthanoid staining for scanning electron misrosopy of biological objects / I.A. Novikov, A.M. Subbot, A.A. Fedorov, I.G. Griboedova, E.N. Antonov, I.V. Vakhrushev. // Genes and cells 2015; Vol. 10, N 2, pp. 90–96.
http://genescells.ru/article/supravitalnoe-kontrastirovanie-lantanoidami-dlya-vizualizatsii-strukturyi-biologicheskih-obraztsov-na-skaniruyushhem-elektronnom-mikroskope-2/ - A new method of biological objects supravital staining shown on an example of an anterior cornea epithelium for the subsequent study on a scanning electron microscope // I.A. Novikov, A.M. Subbot, A.A. Fedorov, I.G. Griboedova. // X Conference of Russian Ophthalmologists. Moscow, 2015, p. 194.
https://eyepress.ru/article.aspx?17462 - Development of a new staining method of biological objects for scanning electron microscopy. / I.A. Novikov, A.A. Fedorov, I.G. Griboedova, A.M. Subbot. // 19th International Puschino conference school of young scientists “Biology, the science of XXI century”. Puschino, 2015, p. 45.
http://download.biology21.ru/19_conference_biology21_full.pdf - Possibility of the eye surface functional evaluation in vitro by SEM. / S.E. Avetisov, A.M. Subbot, I.A. Novikov, T.N. Safonova, A.A. Fedorov. // VIII Russian nationwide ophthalmology forum. Moscow, 2015. p. 759–760.
Download PDF - SEM visualization of corneal epithelium through lanthanoid staining based on Ca/Nd isomorphous substitution in Ca-dependent molecular systems / S.E. Avetisov, S.V. Trufanov, I.A. Novikov, A.M. Subbot, A.A. Fedorov. // Vestn oftalmol. 2016; Vol. 132, N 6, p. 11–19. DOI: 10.17116/oftalma2016132611-19
https://www.mediasphera.ru/issues/vestnik-oftalmologii/2016/6/10042465X2016061011/annotation - Visualization of mesenchymal stromal cells in the 2D and 3D cultures by scanning electron microscopy with lanthanoid staining / I.A. Novikov, I.V. Vakhrushev, E.N. Antonov, K.N. Yarigin, A.M. Subbot. // Cell technologies in biology and medicine. 2016; N4, pp. 248–253.
https://elibrary.ru/item.asp?id=28825547 - Fast and easy method of lanthanoid staining for visualization of cellular ultrastructure and spatial arrangement / Novikov I.A., Subbot A.M., Kiryushchenkova N.P., Nesterova T.V., Gabashvili A.N., Sitnikov A.V., Bursov A.I. // AIP Conference Proceedings 2016; Vol. 1748, Iss. 1, 020009.
DOI: 10.1063/1.4954343
https://aip.scitation.org/doi/10.1063/1.4954343 - Fast and easy method of lanthanoid staining for visualization of cellular ultrastructure and spatial arrangement / Novikov I.A., Subbot A.M., Kiryushchenkova N.P., Nesterova T.V., Gabashvili A.N., Sitnikov A.V., Bursov A.I. // V International Scientific Conference STRANN 2016, Saint Petersburg, 2016, p. 134–136.
https://www.researchgate.net/publication/315787308_Fast_And_Easy_Method_Of_Lanthanoid_Staining_For_Visualization_Of_Cellular_Ultrastructure_And_Spatial_Arrangement - New information content of “old devices” — lanthanoid staining of biological specimens for SEM / A. M. Subbot, I. A. Novikov, T. V. Nesterova, Chebotar’ I.V. // III Internatinal conference of the young scientists, Novosibirsk, 2016, pp. 93–97.
https://openbio.ru/openbio_tezis_2016.pdf - Patent 2015117678/15, 12.05.2015. A method of biological sample preparation for scanning electron microscopy study / I.A. Novikov, A.A. Fedorov, A.M. Subbot, I.G. Griboedova. // Russian Patent № 2578977, 2016. Bulletin #9.
http://www1.fips.ru/fips_servl/fips_servlet?DB=RUPAT&DocNumber=2578977&TypeFile=html - Lanthanoid Staining as a Fast Technology of Preparing Microbiological Specimens for Scanning Electron Microscopy / I.V. Chebotar, I.A. Novikov. A.M. Subbot, N.A. Mayansky. // Sovremennye tehnologii v medicine 2017; 9(3): 23–30, DOI: 10.17691/stm2017.9.3.03
http://www.stm-journal.ru/ru/numbers/2017/3/1363 - Visualization of mesenchymal stromal cells in 2d and 3d-cultures by scanning electron microscopy with lanthanide contrasting / Novikov I.A., Subbot A.M., Vakhrushev I.V., Yarygin K.N., Antonov E.N. // Bull Exp Biol Med 2017; Т. 162, № 4, с. 558–562.
DOI: 10.1007/s10517-017-3659-4
https://link.springer.com/article/10.1007%2Fs10517-017-3659-4 - A new look on the ultrastructure of the cell adhesion complex of the corneal epithelium / I.A. Novikov, A.M. Subbot, S.V. Trufanov, L.Yu. Tekeeva. // Point of view. East-West. 2017; N 1, pp. 64–66.ttps://eyepress.ru/article.aspx?23877
- New staining method development for biological samples for scanning electron microscopy / A.M. Subbot, A.A. Fedorov, I.G. Griboedova. // All-Russian youth conference “Experimental and theoretical biophysics`17”, Puschino, 2017, pp. 13-14.
Download PDF